- Ferulic acid
-
Ferulic acid 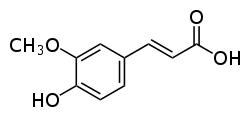 (E)-3-(4-hydroxy-3-methoxy-phenyl)prop-2-enoic acidOther names2-propenoic acid, 3-(4-hydroxy-3-methoxyphenyl)-
(E)-3-(4-hydroxy-3-methoxy-phenyl)prop-2-enoic acidOther names2-propenoic acid, 3-(4-hydroxy-3-methoxyphenyl)-
ferulic acid
3-(4-hydroxy-3-methoxyphenyl)-2-propenoic acid
3-(4-hydroxy-3-methoxyphenyl)acrylic acid
3-methoxy-4-hydroxycinnamic acid
4-hydroxy-3-methoxycinnamic acid
(2E)-3-(4-hydroxy-3-methoxyphenyl)-2-propenoic acid
Ferulate
Coniferic acid
trans-ferulic acid
(E)-ferulic acidIdentifiers CAS number 1135-24-6 
PubChem 445858 ChemSpider 393368 
DrugBank DB07767 ChEBI CHEBI:17620 
ChEMBL CHEMBL32749 
Jmol-3D images Image 1 - COc1cc(ccc1O)/C=C/C(=O)O
Properties Molecular formula C10H10O4 Molar mass 194.18 g/mol Exact mass 194.057909 u Melting point 168-172 °C
 acid (verify) (what is:
acid (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Ferulic acid is a hydroxycinnamic acid, a type of organic compound. It is an abundant phenolic phytochemical found in plant cell wall components such as arabinoxylans as covalent side chains. It is related to trans-cinnamic acid. As a component of lignin, ferulic acid is a precursor in the manufacture of other aromatic compounds. The etymology is from Ferula, referring to the giant fennel (Ferula communis).
Contents
Occurrence in nature
Ferulic acid is found in the seeds of coffee, apple, artichoke, peanut, and orange, as well as in both seeds and cell walls of commelinid plants (such as rice, wheat, oats, and pineapple). It can be extracted from wheat bran and maize bran using concentrated alkali.
Açaí oil, obtained from the fruit of the açaí palm (Euterpe oleracea), is rich in ferulic acid (101 +/- 5.9 mg/kg).[1]
Metabolism
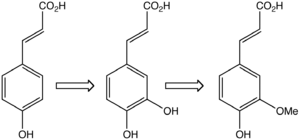 In plants, ferulic acid (right) is derived from phenylalanine, which is converted to 4-hydroxycinnamic acid (left) and then caffeic acid.
In plants, ferulic acid (right) is derived from phenylalanine, which is converted to 4-hydroxycinnamic acid (left) and then caffeic acid.
Biosynthesis of ferulic acid is by the action of the enzyme O-methyl transferase on caffeic acid.[1]It is biosynthesised from caffeic acid.
Ferulic acid, together with dihydroferulic acid, is a component of lignocellulose, serving to crosslink the lignin and polysaccharides, thereby conferring rigidity to the cell walls.[2]
It is an intermediate in the synthesis of monolignols, i.e., the monomers of lignin, and is also used for the synthesis of lignans.
Ferulic acid is converted by certain strains of yeast (notably strains of yeast used in brewing of wheat beers, such as Saccharomyces delbrueckii) to 4-vinyl guaiacol which gives beers such as Weissbier and Wit their distinctive "clove" flavour.
Bio-medical considerations
Ferulic acid, like many phenols, is an antioxidant in vitro in the sense that it is reactive toward free radicals such as reactive oxygen species (ROS). ROS and free radicals are implicated in DNA damage, cancer, accelerated cell aging. Animal studies and in vitro studies suggest that ferulic acid may have direct antitumor activity against breast cancer[2] and liver cancer.[3] Ferulic acid may have pro-apoptotic effects in cancer cells, thereby leading to their destruction.[3] Ferulic acid may be effective at preventing cancer induced by exposure to the carcinogenic compounds benzopyrene[4] and 4-nitroquinoline 1-oxide.[5] Note that these are not randomized controlled trials done with human participants, and therefore, the results of these studies may not be directly applicable to human use.
If added to a topical preparation of ascorbic acid and vitamin E, ferulic acid may reduce oxidative stress and formation of thymine dimers in skin.[6]
Applications
As a precursor to vanillin
Ferulic acid, being highly abundant, may be useful as a precursor in the manufacturing of vanillin, a synthetic flavoring agent often used in place of natural vanilla extract.[7] However, biotechnological processes may be the most efficient method to use ferulic acid as a precursor,[8] and as such, research is still ongoing.
Mass spectrometry
It is used as a matrix for proteins in MALDI mass spectrometry analyses.[9]
Bitterness masker
Kraft Foods has patented the use of sodium ferulate to mask the bitter aftertaste of the artificial sweetener acesulfame potassium.[3]
See also
- Caffeic acid
- Coumaric acid
- Diferulic acids
- Sodium ferulate, a ferulic acid salt
References
- ^ Pacheco-Palencia LA, Mertens-Talcott S, Talcott ST (Jun 2008). "Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Acai (Euterpe oleracea Mart.)". J Agric Food Chem 25 (56): 4631–6. doi:10.1021/jf800161u. PMID 18522407.
- ^ Iiyama, K.; Lam, T B.-L.; Stone, B. A. "Covalent Cross-Links in the Cell Wall" Plant Physiology, 1994 volume 104, pp. 315-320.doi:10.1104/pp.104.2.315
- ^ United States Patent 5,336,513
- a Shahadi, Fereidoon; Naczk, Marian (2004). Phenolics in food and nutraceuticals. Florida, USA: CRC Press LLC. p. 4. ISBN 1-58716-138-9.
- a
 Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: potential mechanisms of action. Breast Cancer Res. 2004; 6(2: R63-74. Epub 2003 Dec 15; PubMed Full text at PMC: 14979919
Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: potential mechanisms of action. Breast Cancer Res. 2004; 6(2: R63-74. Epub 2003 Dec 15; PubMed Full text at PMC: 14979919 - a b Role of NADPH oxidase-mediated generation of reactive oxygen species in the mechanism of apoptosis induced by phenolic acids in HepG2 human hepatoma cells. Arch Pharm Res. 2005 Oct; 28(10): 1183-9; PubMed
- a Protective effects of ellagic acid and other plant phenols on benzo[a]pyrene-induced neoplasia in mice. Carcinogenesis. 1983 Dec; 4(12): 1651-3; PubMed
- a Chemopreventive effects of ferulic acid on oral and rice germ on large bowel carcinogenesis. Anticancer Res. 1999 Sep-Oct; 19(5A): 3775-8; PubMed
- a Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J Invest Dermatol. 2005 Oct; 125(4): 826-32; PubMed
- a Ferulic acid: an antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit Rev Biotechnol. 2004; 24(2-3): 59-83; PubMed
- a Biotechnological production of vanillin. Appl Microbiol Biotechnol. 2001 Aug; 56(3-4): 296-314; PubMed
- a Beavis, R. C.; Chait, B. T., Cinnamic Acid Derivatives as Matrices for Ultraviolet Laser Desorption Mass Spectrometry of Proteins. Rapid Commun. Mass Spectrom. 1989, 3, 432-435; doi:10.1002/rcm.1290031207 PMID 2520223
Hydroxycinnamic acids Caffeic acid | Cichoric acid | Cinnamic acid | Coumaric acid | Diferulic acid | Ferulic acid | Plicatins A and B | Sinapinic acidGlycosides Tartaric acid esters Caftaric acid | Coutaric acid | Fertaric acidOthers Grape reaction productCategories:- Antioxidants
- Hydroxycinnamic acids
- Bitter-masking compounds
- Phenolic compounds in wine
Wikimedia Foundation. 2010.